Scientist, Artist, Developmental Biologist
Paula Cechmanek
About Me
I completed a PhD in Neuroscience at the University of Calgary and am a developmental biologist at heart! Using the developing visual system of tadpoles and zebrafish, I ask questions about how neurons send out axons that navigate through the brain to connect to their targets, and how developing tissues undergo complex shape changes (morphogenesis) to acquire their mature structure. The most exciting thing in my job is using transgenic reporter lines to observe cells migrate in living embryos (in vivo time-lapse microscopy) and making beautiful science figures (amateur artist in me).
- Developmental Biology
- Cell to Cell Signalling
- In Vivo Imaging
- Progenitor Cell Specification
- Rocky Mountain Skiing and Hiking
- Oil Painting

See How We're Learning to See.
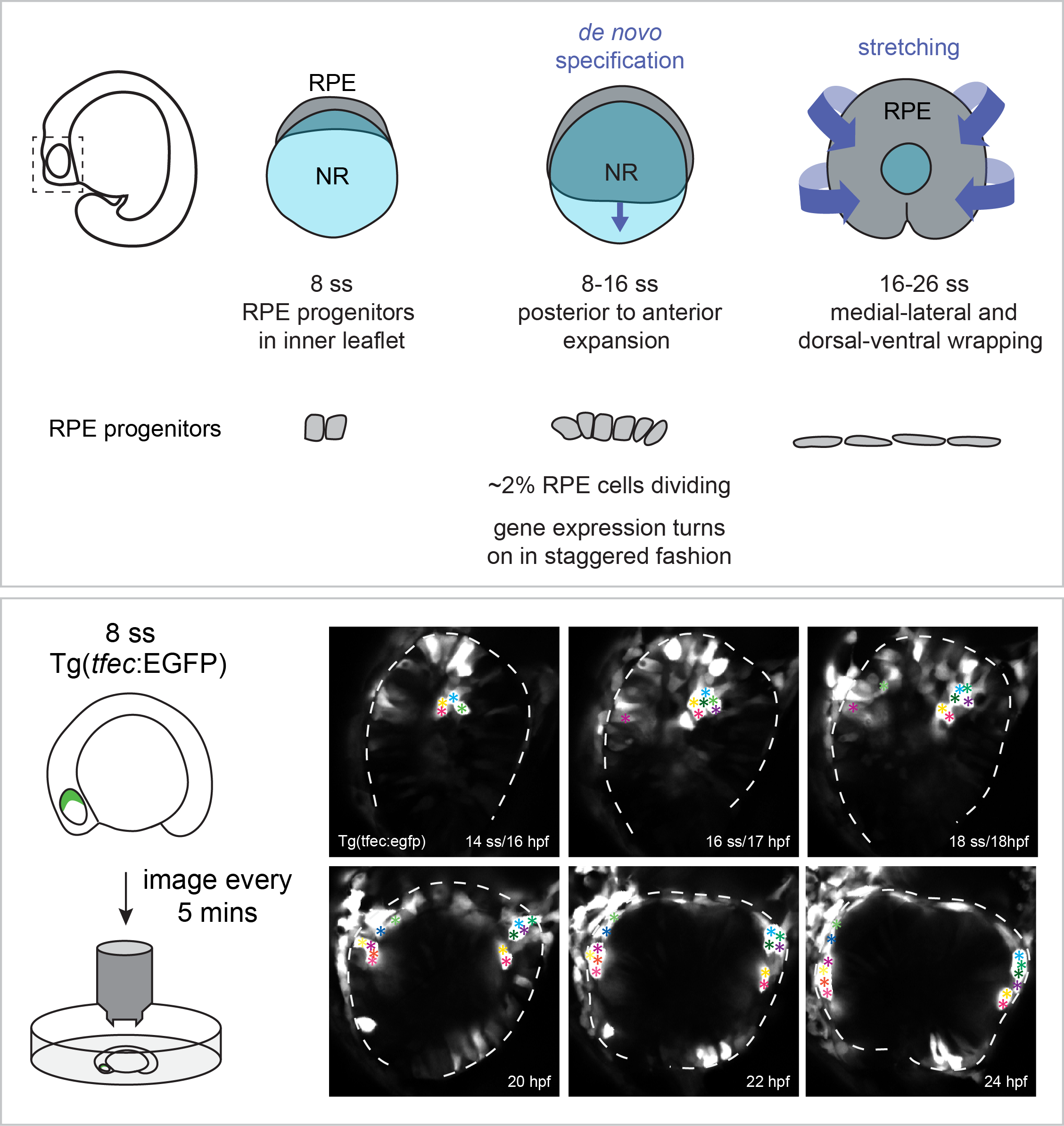
Retinal pigment epithelium expansion around the neural retina occurs in two separate phases with distinct mechanisms.
The retinal pigment epithelium (RPE) is a specialized monolayer of epithelial cells that forms a tight barrier surrounding the neural retina. RPE cells are indispensable for mature photoreceptor renewal and survival, yet how the initial RPE cell population expands around the neural retina during eye development is poorly understood.
Cechmanek, P.B. and McFarlane, S. (2017) Developmental Dynamics. 246: 598-609.
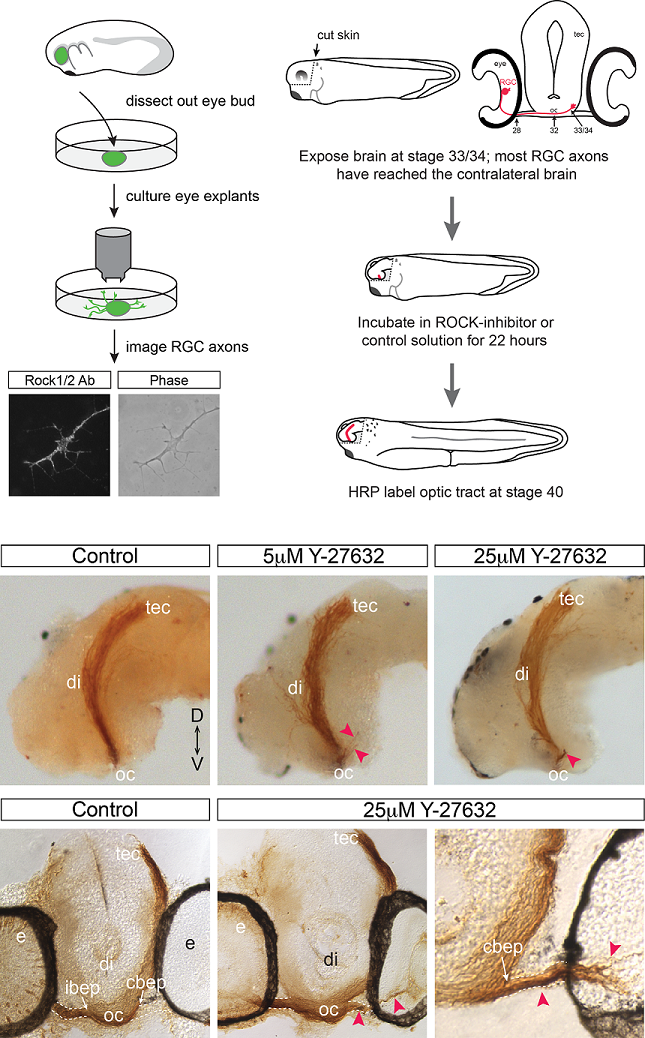
Rho kinase is required to prevent retinal axons from entering the contralateral optic nerve.
To grow out to contact target neurons an axon uses its distal tip, the growth cone, as a sensor of molecular cues that help the axon make appropriate guidance decisions at a series of choice points along the journey. In the developing visual system, the axons of the output cells of the retina, the retinal ganglion cells (RGCs), cross the brain midline at the optic chiasm. Shortly after, they grow past the brain entry point of the optic nerve arising from the contralateral eye, and extend dorso-caudally through the diencephalon towards their optic tectum target. Using the developing visual system of the experimentally amenable model Xenopus laevis, we find that RGC axons are normally prevented from entering the contralateral optic nerve. This mechanism requires the activity of a Rho-associated kinase, Rock, known to function downstream of a number of receptors that recognize cues that guide axons. Pharmacological inhibition of Rock in an in vivo brain preparation causes mis-entry of many RGC axons into the contralateral optic nerve, and this defect is partially phenocopied by selective disruption of Rock signaling in RGC axons. These data implicate Rock downstream of a molecular mechanism that is critical for RGC axons to be able to ignore a domain, the optic nerve, which they previously found attractive.
Cechmanek, P.B., Hehr, C.L. and McFarlane, S. (2015) Molecular and Cellular Neuroscience. 69: 30-40.
Spatially regulated Sema6a and Plxna2 mediated repulsion within the developing eye promotes eye vesicle cohesion.
Organs are generated from collections of cells that coalesce and remain together as they undergo a series of choreographed movements to give the organ its final shape. We know little about the cellular and molecular mechanisms that regulate tissue cohesion during morphogenesis. Extensive cell movements underlie eye development, starting with the eye field separating to form bilateral vesicles that go on to evaginate from the forebrain. What keeps eye cells together as they undergo morphogenesis and extensive proliferation is unknown. Here, we show that plexina2 (Plxna2), a member of a receptor family best known for its roles in axon and cell guidance, is required alongside the repellent semaphorin 6a (Sema6a) to keep cells integrated within the zebrafish eye vesicle epithelium. sema6a is expressed throughout the eye vesicle, whereas plxna2 is restricted to the ventral vesicle. Knockdown of Plxna2 or Sema6a results in a loss of vesicle integrity, with time-lapse microscopy showing that eye progenitors either fail to enter the evaginating vesicles or delaminate from the eye epithelium. Explant experiments, and rescue of eye vesicle integrity with simultaneous knockdown of sema6a and plxna2, point to an eye-autonomous requirement for Sema6a/Plxna2. We propose a novel, tissue-autonomous mechanism of organ cohesion, with neutralization of repulsion suggested as a means to promote interactions between cells within a tissue domain.
Ebert, A.M., Childs, S.J., Hehr, C.L., Cechmanek, P.B. and McFarlane, S. (2014) Development. 141: 2473-2482.
Dynamic expression of axon guidance cues is controlled by FGF signaling.
Axons are guided to their targets by molecular cues expressed in their environment. How is the presence of these cues regulated? Although some evidence indicates that morphogens establish guidance cue expression as part of their role in patterning tissues, an important question is whether morphogens are then required to maintain guidance signals. We found that fibroblast growth factor (FGF) signaling sustains the expression of two guidance cues, semaphorin3A (xsema3A) and slit1 (xslit1), throughout the period of Xenopus optic tract development. With FGF receptor inhibition, xsema3A and xslit1 levels were rapidly diminished, and retinal ganglion cell axons arrested in the mid-diencephalon, before reaching their target. Importantly, direct downregulation of XSema3A and XSlit1 mostly phenocopied this axon guidance defect. Thus, FGFs promote continued presence of specific guidance cues critical for normal optic tract development, suggesting a second later role for morphogens, independent of tissue patterning, in maintaining select cues by acting to regulate their transcription.
Atkinson-Leadbeater, K., Bertolesi, G.E., Hehr, C.L., Webber, C.A., Cechmanek, P.B. and McFarlane, S. (2010) Journal of Neuroscience 30(2): 685-693.
Check out some of my presentations...
Eye vesicle morphogenesis in the developing zebrafish embryo requires Sema6d signaling.
10th European Zebrafish Conference: Budapest, Hungary, July 3-7, 2017.
Sema6d facilitates epithelial flow during optic cup morphogenesis.
Alberta Vision Network: Edmonton, AB, June 5, 2017. Awarded Best Trainee Talk.
Understanding the molecular mechanisms of retinal pigment epihelium development.
Ophthalmology and Visual Sciences Research Day: Calgary, AB, Jan 26, 2015. Awarded Best Basic Sciences Talk.
ROCK keeps retinal ganglion cell axons on track.
14th International Xenopus Conference: Giens Peninsula, France, Sept 9-13, 2012.